The solvent extraction electro-winning process (SX-EW) is a hydrometallurgical means of extracting, purifying, and concentrating metals. SX-EW has continued to demonstrate successful use in effectively extracting and recovering copper from low grade and oxide ores.
As a result, SX-EW has quickly become a staple in the copper industry; SX-EW is currently responsible for producing around 20% of the world’s copper (see the infographic on copper production). This demonstrated success is now pushing the process into a number of other markets as well.
How SX-EW Works
SX-EW, sometimes called Leach Solvent Extraction – Electrowinning for its joining with leaching techniques, takes place in two parts. The first step, solvent extraction (SX), serves to extract a target component from an impregnated solution (obtained through leaching). The second step, electrowinning (EW), is an electroplating process that allows the extracted metal to be electrowon. In this step, an electric current is passed through a bath of the extracted material, causing the target metal to deposit onto metal plates, resulting in a pure form of the metal that can be removed from the plates and move on to subsequent processing and sale. This process is illustrated below for a copper production setting.
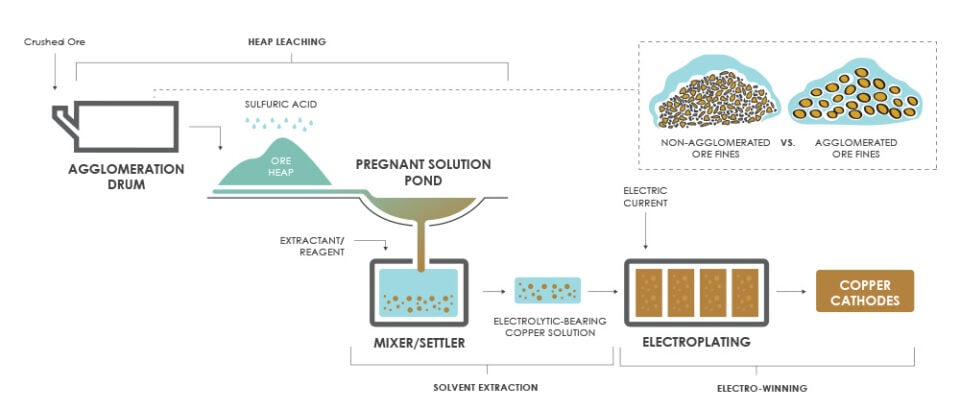
Solvent extraction has been used independently for a long time. Recent advancements however, particularly in the development of improved extractants/reagents, have brought a resurgence of interest to the process.
Combined with electrowinning, the united processes create a highly effective method for extracting and purifying metals that might otherwise be uneconomical to process, or that might have previously been regarded as obsolete due to traditional processing methods being inadequate.
Leaching: A Precursor to SX-EW
SX-EW is always preceded by a leaching process, which produces the impregnated solution that serves as a feedstock for solvent extraction. For this reason, it is often also referred to as leach solvent extraction electrowinning, or L-SX-EW.
An effective leaching step is critical to the success of the operation, as this sets the stage for the amount of recovery possible. A variety of leaching methods exist, with the primary approach being heap leaching, a process in which crushed ore is piled onto a heap irrigated with a leachate. As the leachate percolates through the heap, it picks up the target metal and is collected as a pregnant liquor. ALTA Metallurgical Services Managing Director and Metallurgical Consultant stated in International Mining that “the ability to use heap leaching, together with SX/EW to go from ore to high grade metal at the mine-site, makes it particularly attractive to aspiring juniors.”
When leached via heap leaching, the crushed ore is often first agglomerated in an agglomeration drum (also referred to as an ore drum or agglomerator) to improve the uniformity of the ore fines, particularly when an excess of fines or clay is present in the material.

The agglomeration step maximizes leachate percolation through the heap, as seen in the illustration below.
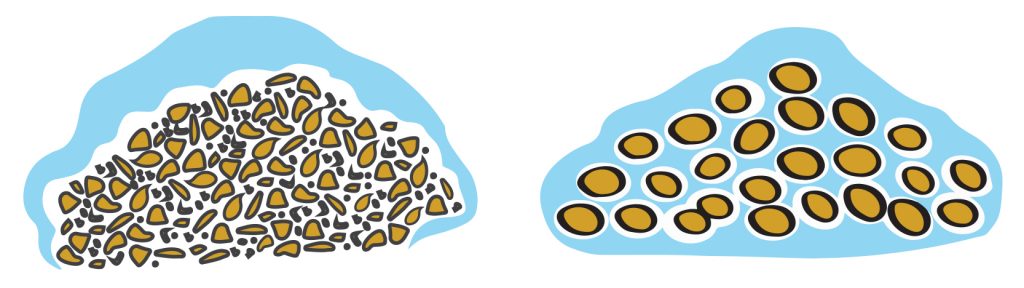
Left: Ore fines that have not been agglomerated lack uniformity, preventing the leachate from effectively percolating through the heap.
Right: Ore fines that have been agglomerated are more uniform in shape and size, allowing for improved percolation of the leachate through the heap.
Factors Pushing SX-EW into New Markets
A number of factors are causing SX-EW to see widespread use. Among them:
- Advancements in extractants/reagents have made the process more effective and more viable in many settings.
- High-grade ore deposits are diminishing, resulting in a need for an economic technology for extracting metals from low-grade ore sources.
- SX-EW offers lower capital and operating costs than smelting.
- SX-EW is attractive because it offers easy operation and requires minimal supervision.
In International Mining, Gustavo Diaz and his colleagues at Tecnicas Reunidas commented on the growing trend:
“The low capital and operating costs of SX plants together with the easy operation and the production of top quality electrolytic metals close to the mine site make the economics of the SX processes very attractive, being suitable and feasible in the range of small to medium capacities, where conventional smelting process is not applicable.”
Here, we’ll look at some of the recent advancements in how SX-EW has been applied to new markets.
Nickel & Cobalt
As deposits of nickel-bearing sulphide ores become less economic to mine, it is becoming more common to mine nickeliferous lateritic ores instead, which are closer to the surface and subsequently, more cost effective to mine. These lateritic ores, also a major source of cobalt, make up about 70% of the Earth’s known land-based nickel supply. However, this abundant source of nickel requires more processing and therefore incurs greater processing costs compared to sulphide ores.² Combined with the copper industry’s proven success, SX-EW has seen growing interest in the nickel industry.
SX-EW’s application in the nickel industry has also promoted its use with cobalt, as the two base metals often occur together. Nickel is a major source of cobalt, but the close placement of nickel and cobalt on the periodic table makes them difficult to separate from each other, due to their similar properties. Advancements in extractants have allowed solvent extraction (SX) to significantly improve upon the separation of cobalt from nickel, further expanding SX-EW in this market.³
Zinc
The traditional approach to processing zinc, known as the roast-leach-electrowin (RLE) process has long been used to effectively process sulphide ores, but has limitations when it comes to more complex sulphide ores, and is not capable of processing other ore types. Additionally, the RLE approach produces SO2 gas, which requires fixation. However, advancements in solvent extraction have allowed this technology to be effectively applied in zinc processing prior to electrowinning.
The SX-EW approach to processing zinc allows more complex ores, as well non-sulphide zinc ores to be effectively treated. Similarly, SX-EW has been widely applied to extracting zinc as an impurity in other base metal processing operations. This approach can be used with both primary and secondary zinc materials (zinc residues and furnace dusts, zinc scrap/recycled zinc).¹
The Skorpion Zinc Mine, located in Namibia and owned by Vedanta Resources, was the first commercial application of SX-EW in a primary zinc processing setting. In 2014, Horsehead opened an SX-EW plant in Mooresboro, NC for secondary zinc processing (primarily EAF dust).
Additional Applications
In addition to these materials, SX-EW is also gaining traction in a variety of other markets as well, including gold, silver, uranium, and rare earths.
Many are beginning to utilize this technology as a way to treat mine tailings and wastes, or other sources that contain valuable metals, but not in high enough concentration to economically process via traditional methods.
Conclusion
In a time when high grade ores are diminishing amidst a booming global population demanding more metals, never has it been more important to maximize our available resources.
The demonstrated success of the solvent extraction – electrowinning process in the copper industry for oxide and low grade ores has pushed it into a variety of other metal markets. As a result, SX-EW (and its joining with leaching techniques), has become a critical tool in processing complex ores, and ore sources that would otherwise have been considered uneconomic to process.
FEECO provides custom agglomeration drums for use in the heap leaching process, as well as the necessary handling equipment to support the process. With agglomeration drums in some of the world’s largest and most environmentally advanced mines, FEECO agglomeration drums are relied upon by the world’s foremost mining companies. For more information, contact us today!
Sources
- Cole, P.M., and K.C. Sole. “Solvent Extraction in the Primary and Secondary Processing of Zinc.” The Journal of the South African Institute of Mining and Metallurgy (2002): 451-56. Web. Mar. 2017.
- Bacon, G. and Mihaylov, I. “Solvent Extraction as an Enabling Technology in the Nickel Industry.” The Journal of the South African Institute of Mining and Metallurgy (2002): 435-44. Web. Mar. 2017.
- Lakshmanan, Vaikuntam Iyer, Raja Roy, and V. Ramachandran. Innovative Process Development in Metallurgical Industry. N.p.: Springer International Publishing AG Switzerland, 2016.

