In the complex production required to transform spodumene concentrate into lithium compounds such as lithium carbonate or lithium hydroxide, calcination plays a key role in not one, but two parts of the process, making it an essential technique in efforts to exploit this increasingly important lithium ore.
While the beneficiation of lithium from brines has long been the primary focus of commercial efforts, spodumene – a pegmatite ore – is becoming more desirable as a result of its high lithium content and the exploding demand for lithium-ion batteries.
Calcination, often referred to as roasting in this setting, is one of the more common processing techniques used throughout the extraction of lithium from spodumene.
What is Calcination?
Calcination is a thermal processing technique used throughout a variety of industries to instigate a chemical reaction or physical change in a material. Most commonly, it refers to the chemical dissociation of components within a material, such as the separation of calcium carbonate to produce calcium oxide and carbon dioxide.
Calcination typically occurs in an inert environment. However, it’s important to recognize that the term is widely used to describe all manner of thermal processes.
In the production of lithium compounds from spodumene concentrate, calcination is utilized to carry out two separate steps in the process: decrepitation and acid roasting.
Decrepitation
Spodumene ore naturally occurs in the crystal structure of monoclinic alpha form (α-form). In order to extract lithium from the ore via the leaching process, however, the ore’s crystal structure must be in the tetragonal beta form (β-form).¹ This conversion is achieved through decrepitation, or the shattering of the crystal structure.
Facilitated by calcination, decrepitation expands the spodumene’s crystal structure by about 30%, ultimately allowing the otherwise chemically inert ore to react with sulfuric acid.¹
In processing spodumene concentrate, calcination causes decrepitation at temperatures between 1075 – 1100 C. Temperature control during calcination is crucial to the success of the process; if temperatures are allowed to approach anywhere near 1400 C, undesirable formations between the alpha spodumene and other silicates can occur (referred to as eutectics).¹
Since the α-spodumene ore is not sensitive to products of combustion, a direct-fired rotary kiln is used to process the material in this setting. A direct-fired kiln utilizes direct contact between the material and process gas to thermally process the ore. The kiln is set at a slight angle in order to allow gravity to assist in moving material through the rotating drum.

3D Model of a FEECO Direct-fired Kiln
Acid Roasting
Once the material has been decrepitated, or converted to beta-phase spodumene (β-form), an additional calcination step is required. The concentrate, now in β-form, is first mixed with sulfuric acid (a step referred to as sulfuric acid digestion) and fed to a separate rotary kiln. This acid roasting step utilizes a kiln that operates at much lower temperatures – typically around 250 C.¹
The goal of the acid roasting step is to allow the lithium to be extracted from the mixture as water-soluble lithium sulfate, which is amenable to leaching. Through leaching, the lithium sulfate can then be converted into market-ready compounds lithium carbonate or lithium hydroxide.
Unlike the kiln used for decrepitation, this kiln is of the indirect configuration, often referred to as a calciner. The indirect configuration is used because beta-phase spodumene cannot be exposed to the products of combustion.
Indirect-fired kilns are externally heated in order to keep the material and products of combustion separate. Material, heated through direct contact with the shell, rolls along the drum’s interior, exposing fresh material as it tumbles to promote even heat distribution throughout the bed, sometimes with the help of bed disturbers.
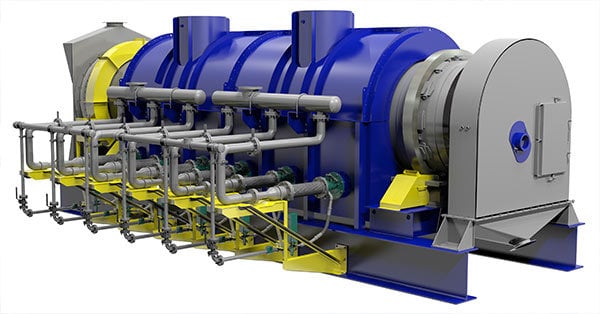
3D Model of a FEECO Rotary Calciner
Spodumene Calcination Testing
Despite the process of recovering lithium from spodumene being fairly well established, testing is often an integral step in achieving maximum lithium recovery.
Testing lithium recovery from spodumene in a facility such as the FEECO Innovation Center allows process variables to be worked out and can produce an array of samples for further testing.
Process Engineers in the Innovation Center have various kilns at their disposal to conduct testing at batch and continuous pilot scale for both direct-fired applications (decrepitation) and indirect applications (acid roasting).
Various process data points can be tested to find the optimal combination for lithium recovery. In testing spodumene, testing typically centers around the following variables:
- Retention/Residence time
- Temperature
- Gas sampling & analysis
- Energy requirements
Conclusion
Calcination is an essential tool in the conversion of spodumene concentrates to lithium compounds, with two key roles in the process. Through calcination, both decrepitation and acid roasting can be achieved in the effort to produce lithium carbonate and lithium hydroxide for use in lithium-ion batteries or other applications.
FEECO is a leader in custom thermal processing equipment. We can provide custom rotary calciners for use in the lithium extraction process, be it for decrepitation or acid roasting. All FEECO equipment is engineered and fabricated to the highest quality with the characteristics of the material in mind to ensure a reliable, long-lasting thermal processing solution. We also offer a unique testing facility to aid in process development. For more information, contact us today!

