Activated alumina is a form of aluminum oxide (Al2O3) with a myriad of industrial uses. A recent industry report by Grand View Research estimates the activated alumina market will see a CAGR of 5.4% up to 2025, largely as a result of its use in water treatment applications, which continue to grow in importance, as well as its use as a catalyst in the oil and gas industry.
Activated alumina exhibits a number of characteristics that make it ideal in many industrial process settings. This includes a high crush strength, resistance to thermal shock, resistance to chemical attack, and more. The characteristic that has pushed activated alumina to the forefront of many applications is its capability as an adsorbent, courtesy of its high porosity and surface area.
Producing the desired characteristics of an activated alumina product is achieved through a carefully controlled production process.
Production of Activated Alumina
In most cases, activated alumina starts out as an aluminum hydroxide (gibbsite, boehmite, etc.) – a material obtained through a series of chemical reactions in the Bayer process used to transform bauxite ore into alumina.
Activated Alumina Calcination
Once the aluminum hydroxide has been produced, it is thermally treated via calcination in a rotary kiln. This calcination step serves to dehydrate, or remove bound moisture from the aluminum hydroxide in order to produce alumina, or aluminum oxide (Al2O3).
Activation, a phase in which the alumina structure becomes highly porous, occurs within a specific temperature range, with process parameters such as residence time and temperature profile used to control the properties of the end product. The makeup of the original bauxite source can also influence the characteristics of the end product.
Activated Alumina Agglomeration
Depending on the intended end use of the activated alumina, agglomeration is also often desirable. This is especially true when working with adsorbents and catalysts.
The agglomeration of activated alumina allows for a high level of customization for specific application requirements. Characteristics that are often controlled through agglomeration include:
- Particle size distribution
- Bulk density
- Crush strength
- Amount of attrition/potential for dust generation
- Flowability
- And more…
There are many ways to produce activated alumina “beads,” as they are often called, with some options including the use of an agglomerator, pin mixer, disc pelletizer, or combination thereof.
Activated Alumina Testing
For many reasons, testing is often a critical step in the success of an activated alumina product that performs as desired. It is often necessary to test the thermal processing aspects of activated alumina production using batch- and pilot-scale kilns. Additionally, it is often recommended to test the various methods of agglomeration, as well as potential binders in order to gather the process data necessary to produce an agglomerate with the required characteristics.
The FEECO Innovation Center is a testing facility where both thermal and agglomeration testing can be carried out at batch and pilot scale. Continuous process loop testing integrating thermal and agglomeration can also be tested. This unique testing environment allows process and material data to be gathered, and production conditions to be simulated in order to create a process that will produce a product suited for the intended application.
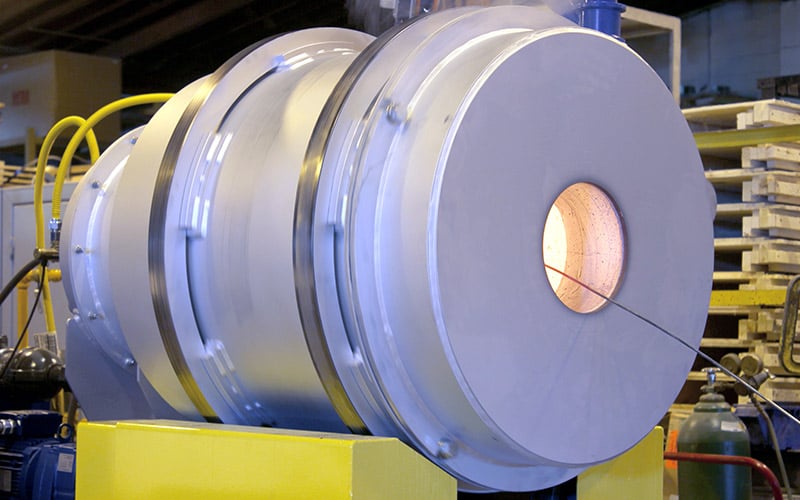
Batch Kiln in the FEECO Innovation Center
Activated Alumina Applications
Much like activated carbon, the high surface area and porosity exhibited by activated alumina allows it to capture and hold on to various types of materials, lending it to be employed as an adsorbent, desiccant, and more. The primary ways in which activated alumina products are used include:
Adsorbents
Activated alumina is a highly effective adsorbent in both gas and liquid applications, and as such, is employed by a number of industries for targeted removal of components from other media.
As an adsorbent, activated alumina is most well known for its use in water filtration applications, where it serves as a cost-effective adsorbent for removing fluoride from water. It is also capable of removing a variety of other contaminants, including arsenic, lead, and sulfur.
Desiccant
Similar to its role as an adsorbent, activated alumina can also adsorb water from air, allowing it to be used as a desiccant; activated alumina can capture and trap water in order to keep things dry, much like silica gel. As a desiccant, activated alumina can adsorb up to 20% of its own weight in water at a relative humidity of 50%.¹
Activated alumina is employed as a desiccant in a wide variety of applications, including the removal of water vapor from gases in industrial settings. Water adsorbed onto the activated alumina can then be desorbed via thermal treatment and the alumina reused.
Catalysts
Activated alumina is also widely used as a catalyst, with roles as the catalyst itself, as well as an inert carrier, or substrate for other catalysts.
As a catalyst, activated alumina is most well known for its role as a Claus catalyst; activated alumina is the most commonly used Claus catalyst in sulfur recovery endeavors at oil and gas refineries.
Conclusion
Due to its capabilities as an adsorbent, desiccant, and catalyst, activated alumina is a valuable tool in many industrial process settings. Produced from the activation of aluminum oxide resulting from the Bayer process via calcination, activated alumina is highly customizable and is often agglomerated to improve performance and handling characteristics. Feasibility testing is often a useful endeavor when working with activated alumina products.
FEECO offers comprehensive testing capabilities for both the thermal treatment and agglomeration of activated alumina, as well as alumina and bauxite. In addition to our process and product development services, we engineer and manufacture custom rotary kilns and agglomeration equipment for all of your activated alumina needs. For more information, contact us today!
SOURCES
1.Kirk-Othmer. Kirk-Othmer Encyclopedia of Chemical Technology, 5th Edition, Volume 2, 5th. John Wiley & Sons, 2004.

